Chemistry frequently concerns compounds that, although not familiar in other settings
outside laboratories, form a part of numerous industrial and research processes. A good example of such a compound
is trifluoromethanesulfonic anhydride, widely abbreviated as Tf₂O. In synthetic organic chemistry, Tf₂O serves as a
very useful reagent with remarkable properties, worth reactivity, and many uses.
This piece will delve into what makes trifluoromethanesulfonic anhydride stand out —
from its chemical composition to its behaviour in reactions, and why chemists turn to it for sophisticated
transformations.
What Is Trifluoromethanesulfonic Anhydride?
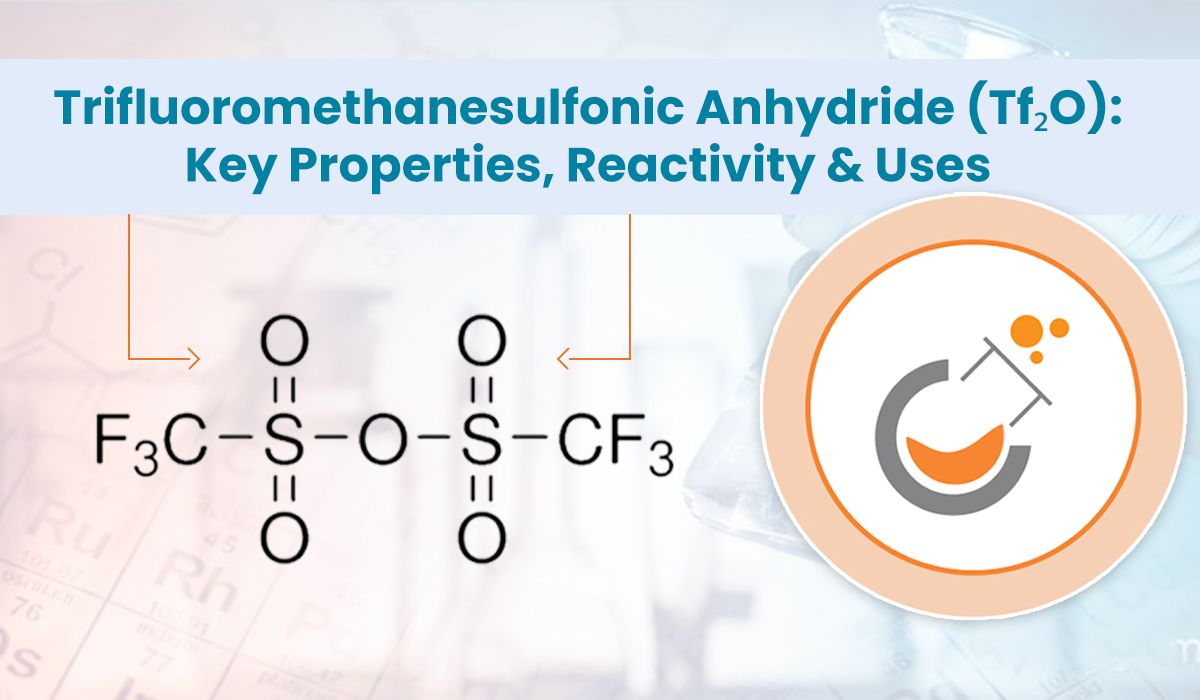
Trifluoromethanesulfonic anhydride is an organofluorine reagent with the chemical
formula (CF₃SO₂)₂O. It is the anhydride of trifluoromethanesulfonic acid (or triflic acid), one of the strongest
known acids.
Trifluoromethanesulfonic anhydride is a pale yellow to colourless liquid at room
temperature with a sharp, pungent smell. Trifluoromethanesulfonic anhydride is moisture-sensitive and readily reacts
with water to form triflic acid. Therefore, it should be handled in dry inert conditions like in a glove box or
under nitrogen or argon protection.
What Is Trifluoromethanesulfonic Anhydride?
Knowledge of the physical properties of trifluoromethanesulfonic anhydride is essential
to handle it safely and efficiently.
Molecular Formula: ₂F₆O₅S₂
Molar Mass: 282.13 g/mol
Appearance: Pale yellow to colorless liquid
Boiling Point: ~81–83°C at 20 mmHg (low pressure)
Density: ~1.68 g/cm³ at 20°C
Solubility: Does not mix with water; soluble in a variety of organic solvents like dichloromethane,
acetonitrile, and ethers
Stability: Moisture-sensitive, hydrolyzes on contact with water to give triflic acid
The intense electron-withdrawing influence of the trifluoromethyl (–CF₃) substituents
helps explain the compound’s high reactivity.
Reactivity of Trifluoromethanesulfonic Anhydride
The reactivity of Tf₂O is due to two crucial reasons:
Strong Electrophilicity – Trifluoromethanesulfonic anhydride sulfur atoms are extremely
electron-deficient, and thus more susceptible to nucleophilic attack.
Good Leaving Groups – The triflate group (–OSO₂CF₃) is a good leaving group in organic
chemistry, allowing smooth conversions.
Some characteristic reaction tendencies are:
Activation of Hydroxyl Groups – Converting alcohols to triflates, much better
leaving groups in substitution or reactions.
Dehydration – Helping to remove water in reactions, particularly in the synthesis
of acid chlorides or ketenes.
Acylation and Alkylation Aid – Making reactivity better in Friedel–Crafts and
related electrophilic substitution reactions.
Cyclization Reactions – Assisting the formation of cyclic structures in
complicated organic syntheses.
Applications of Trifluoromethanesulfonic Anhydride
Due to its special chemical constitution, trifluoromethanesulfonic anhydride has been employed in various applications
in laboratory and industrial chemistry.
1. Triflation of Alcohols and Phenols
One of its most prevalent applications is the transformation of alcohols and phenols into triflates. Triflates are
highly reactive cross-coupling intermediates in Suzuki, Stille, and Heck reactions.
For instance:
Phenol + Tf₂O → Aryl triflate (excellent C–C bond forming compound).
2. Activation of Carboxylic Acids
Tf₂O is also able to convert carboxylic acids to acyl triflates, which are reactive intermediates for nucleophilic
substitution. This is especially beneficial in peptide coupling and in the synthesis of fine chemicals.
3. Dehydration in Organic Synthesis
Trifluoromethanesulfonic anhydride is useful for the removal of water from specific functional groups so that
anhydrides, nitriles, and ketenes can be formed under mild conditions.
4. Catalyst Component
Although Tf₂O is frequently a reagent, it can also be used as part of catalytic systems, specifically when it is mixed
with Lewis acids, improving selectivity in electrophilic processes.
5. Pharmaceutical and Agrochemical Synthesis
Numerous active pharmaceutical ingredients (APIs) and agrochemicals necessitate triflate intermediates in their
synthesis. Tf₂O provides a high-yield route to such prized compounds.
Handling and Safety Precautions
Similar to many potent reagents, trifluoromethanesulfonic anhydride needs to be handled with caution:
Moisture Protection – Always operate under an inert atmosphere to avoid unwanted reaction with water.
Protective Gear – Wear gloves, goggles, and lab coats; do not come into skin contact or inhalation.
Ventilation – Perform all operations in a fume hood.
Storage – Store in sealed containers in dry conditions, away from bases, strong nucleophiles, and moisture.
In case Tf₂O comes into contact with the skin or eyes, wash immediately with large amounts of water and get medical
advice.
Why Chemists Appreciate Tf₂O
To synthetic chemists, having both a very reactive and selectively useful reagent is often elusive.
Trifluoromethanesulfonic anhydride provides:
Reproducible Reactivity – It consistently activates hydroxyl and carboxyl groups.
Mild Conditions – Most reactions can be conducted at low temperatures with high yields.
Versatility – Useful in organic, medicinal, and materials chemistry.
Its function in forming reactive intermediates places Tf₂O at the center of intricate synthetic pathways.
Conclusion
Trifluoromethanesulfonic anhydride is more than an oblong-named chemical — it is a horse-hoe reagent in contemporary organic synthesis. From OH activation to facilitating carbon–carbon bond formation, its special reactivity makes it a chemist’s indispensable tool in research and industry.
When treated with respect and appropriate safety precautions, Tf₂O provides access to effective, high-yielding conversions that are challenging or impossible using less reactive reagents.
No matter the application in pharmaceutical research, agrochemical manufacturing, or materials development, trifluoromethanesulfonic anhydride continues to demonstrate its worth as a foundation reagent in the chemist’s arsenal. Explore more: Triflic Acid Anhydride A Powerful Reagent Reshaping Pharmaceutical Synthesis.





Recent Comments